Now, a bit of background. Viruses are sneaky little buggers and have evolved over time to sneak past your normal immune defences and infiltrate your cells, and from there trick the cell into making more of the virus. Normally this is a bad thing such is the case with HIV or the more common virus, the flu. So what happens, if we take away all the parts that allow a virus to make us sick and give it some specific DNA that a patient might be missing. Then the virus will go, sneak into the persons cells and trick the cell into making proteins from that DNA, but the proteins will be good for the patient and replace an otherwise missing protein.
There are many diseases that are caused by a mutation in the DNA which then means the protein isn't made in the patient, so if we could somehow deliver the protein (or at least the blueprints for the protein, DNA) into the cells then we could replace what was missing.
 |
| Gene therapy using an AAV virus. (Source: Wikipedia) |
Gene therapy has been successfully and safely used to treat many diseases such as chylomicronemia, chronic lymphocytic leukemia, and multiple myeloma and a clever technique has recently been published to advance the use of this technique to treat certain types of blindness.
Inherited forms of retinal diseases afflict 1 in 3000 people worldwide and are primarily the result of mutations encoding proteins in the retina of the eye. Current gene therapy treatment for these diseases involves a needle piercing the eye and penetrating the retina itself to successfully deliver the virus. The diagram below shows just how far back that needle would have to go. The process requires hospitalization, general anaesthetic and runs a real risk of damaging the retina itself. This is because the special viruses usually used in gene therapy (called AAV) cannot get that far back into the eye normally and so need to be injected directly there.
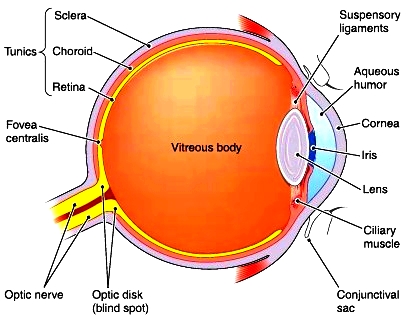 |
| Source: http://encyclopedia.lubopitko-bg.com |
A group from Berkeley University has made use of the high rate of evolution in viruses (because they reproduce so quickly) and used directed evolution to "evolve" a virus that has the capabilities to get that far back in the eye. To do this, they took the standard AAV virus and injected it into the gel layer at the outer eye (the vitreous body in the diagram above), they then waited a while (a week) and collected the cells in the retina of the eye to see if any viruses made it that far back. There were some! So they let these few replicate into huge numbers again and repeated the process and repeated it and repeated it, each time only continuing with the viruses that somehow had the ability to reach the back of the eye, therefore selecting the viruses with the desired ability. At the end of all this they found 48 AAV viruses made into the back of the retina and 2 thirds of them were the same virus, they dubbed it 7m8.
To test if this virus could then be used to treat a disease, they used two mouse models of retinal degeneration caused by malfunctioning proteins in the retina and split each disease model into two groups. In one group they injected the virus, packaged with the DNA required to make the missing/malfunctioning protein, and the other group received the virus by itself without any extra DNA packaged into it. The mice with the 7m8 loaded with DNA had great vision improvements and a protein analysis proved that the retina was in fact producing the previously missing protein.
 |
| Immunostaining for RS1 protein (Dalkara et al. 2013) |
Now you might be thinking? So what? They still need an injection in the eye to get the virus in the first place, and you'd be right! However, injecting into the vitreous gel layer is a simple procedure, done at your local doctors practice, under local anaesthetic and runs very little risk of damaging the retina and this makes it a much more practical solution to those rendered blind from these diseases. Furthermore, this study, as a general concept, paves the way for developing more AAV viruses that are specialised to reach certain tissue or organs.
The study was published on the 12th June in Science Translational Medicine and can be viewed here.
No comments:
Post a Comment